Producing hydrogen through electrolysis
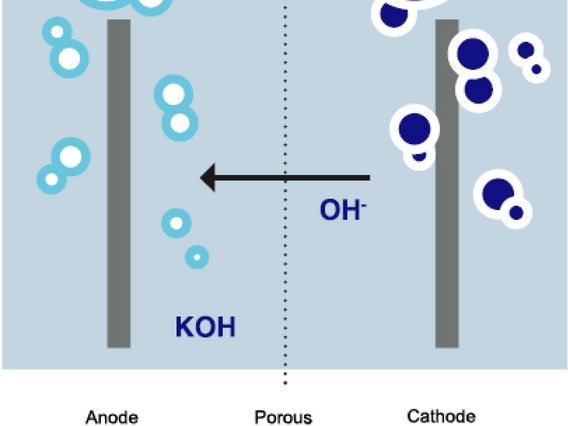
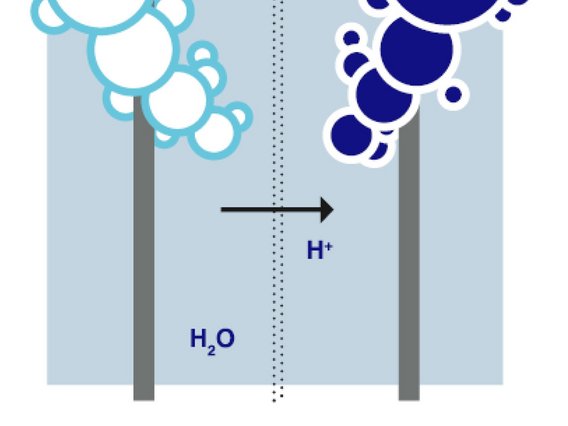
"Electrolysis" refers to the splitting of a chemical compound through the use of an electric current. Electrolysis therefore separates the substances contained in the compound. Electrolysis is carried out in an electrolyser. The process requires two electrodes (anode and cathode), a direct current source and an electrolyte (e.g. pure water or alkaline compounds), i.e. an electrically conductive liquid. As hydrogen (H2) only exists in bound form, it must first be dissolved from this compound. Electrolysis is therefore necessary for the production of hydrogen: In this process, two hydrogen molecules (2H2) and one oxygen molecule (O2) are obtained from two water molecules (2H2O). This form of electrolysis is also known as "water electrolysis".
Hydrogen is a multifaceted topic. As a technology service provider with over 150 years of experience, we are your independent partner for safety, quality and hazard reduction by testing, inspecting and certifying various aspects of hydrogen technology.We are your partner for the development, testing and integration of high-performance electrolysers into your process chains - from the use of smaller systems in research facilities or in the mobility sector to large plants in energy-intensive industries.
With state-of-the-art analysis and measurement methods and competent experts, we are at your side to carry out your project safely and successfully and, if possible, to let you benefit from subsidies. Get in touch with us.