Using hydrogen: What does a fuel cell do?
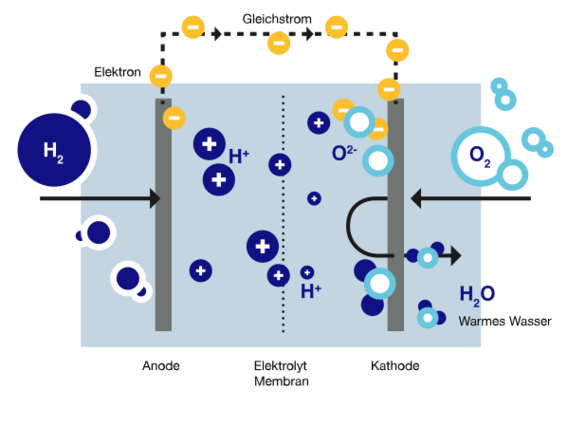
A fuel cell is required to convert hydrogen (H2) into electricity, specifically a hydrogen-oxygen fuel cell. It is often simply referred to as a "hydrogen fuel cell". In the following, "fuel cell" and "hydrogen fuel cell" are used synonymously. In hydrogen fuel cells, hydrogen serves as the fuel and oxygen (O2) as the oxidising agent. By converting chemical energy directly into electrical energy and heat, hydrogen fuel cells are significantly more efficient than conventional power stations.
In combination with a fuel storage system and hydrogen recycling, fuel cell systems enable pollutant-free energy generation. The performance spectrum of hydrogen fuel cells ranges from the sub-kW range of individual cells to the MW range in the form of virtual power plants. The field of application for hydrogen fuel cells ranges from heat and power supply in buildings to off-grid applications and the propulsion of vehicles, aeroplanes and ships. The discussions surrounding the role of hydrogen for e-mobility in particular have brought fuel cells increasingly into focus.