Hydrogen production
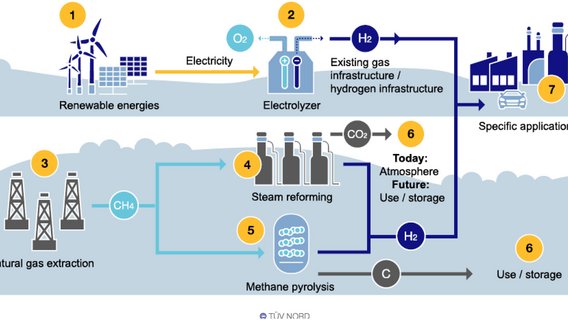
Hydrogen (H2) is the most common element in the universe, present in very large quantities on Earth and contained in almost all organic compounds. At the same time, it only occurs in bound form - the best-known example of this is water (H2O), which is made up of the elements oxygen (O2) and hydrogen. As hydrogen is a secondary energy, primary energy is always required to produce hydrogen. It can be used to store and transport energy.
The choice of primary energy determines whether the hydrogen is produced in an environmentally friendly way. If electricity from renewable energy sources is used entirely, the end product is sustainable hydrogen. This is also known as green hydrogen, as no carbon dioxide (CO2) is emitted during its production. An overview of the different colour categories of hydrogen can be found further down on this page. But how is hydrogen produced? If you want to produce hydrogen, there are a number of processes you can use: With regard to the production of hydrogen, the reforming process and the water electrolysis are very mature. The Kvæner process, hydrogen production from green algae and the production of biohydrogen are still being trialled.
Hydrogen is a topic with many facets - from production, transport and storage to utilisation. With our expertise, know-how and many years of experience, we are an independent partner for safety and hazard prevention by being able to test, inspect and certify various aspects of hydrogen technology.