MDSAP: An audit for five markets
In the past, medical device manufacturers wishing to licence their products in Australia, Brazil, Canada, Japan and the USA had to use individual approval procedures to show compliance with national regulatory requirements. This was both costly and time-consuming.
As an answer to this, the International Medical Devices Regulators Forum (IMDRF) developed a unified program, the Medical Device Single Audit Program (MDSAP), which covers the regulatory requirements of several countries. Since then it has been possible for manufacturers to demonstrate compliance with all these requirements by means of one single audit.
TUV USA Inc. (a member of the TÜV NORD Group) was one of the first certification bodies to be registered by the relevant regulatory authorities and is therefore entitled to perform MDSAP audits.




Benefits of MDSAP certification
- Meet the regulatory requirements in Australia, Brazil, Canada, Japan and the USA with one single audit
- Accelerate market approval [3 countries (Argentina, Singapore and South Korea) have joined the MDSAP program as Affiliate Members]
- Minimise operational disturbance (only one audit)
- Optimum use of regulatory resources
- Less internal work is required
- Reduce audit time and costs
- Improve transparency in the MD sector
- Additional synergy effects possible through inclusion of further audits (ISO 13485 / CE audit)
MDSAP certification process
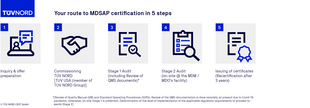
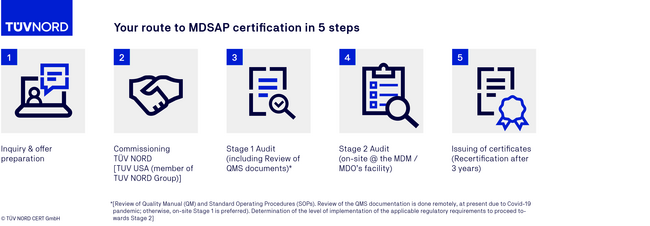
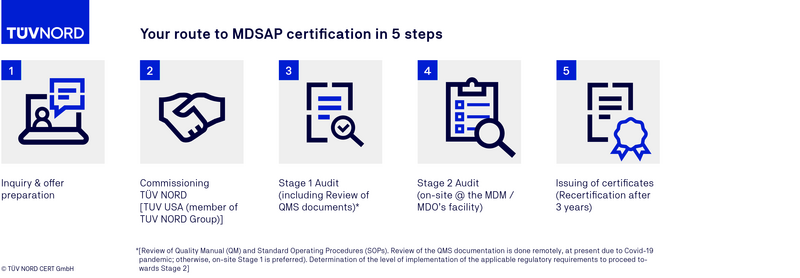
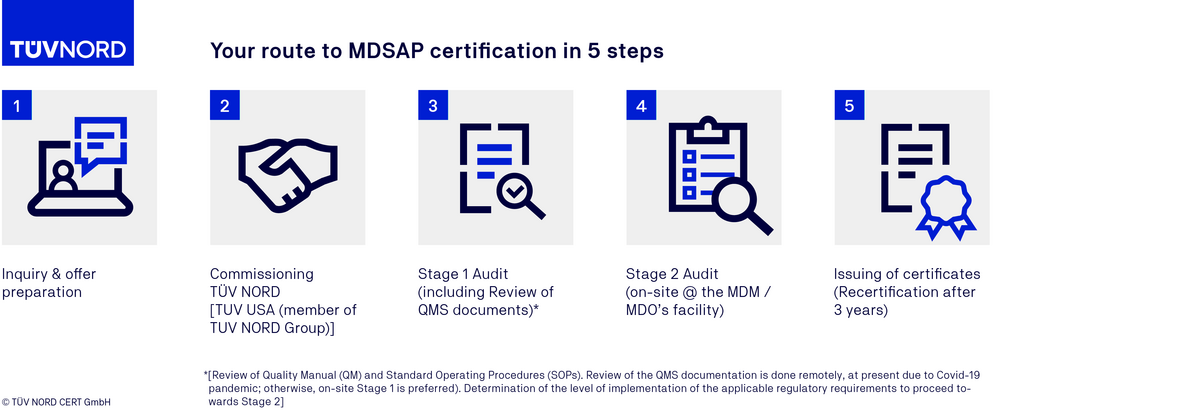
FAQs on the MDSAP audit
MDSAP stands for “Medical Device Single Audit Program” where the specific national requirements of members of the scheme and the requirements of ISO 13485 are all covered by one single audit.
On 1 January 2019, Health Canada, the Canadian Medical Devices Conformity Assessment System (CMDCAS), was replaced by MDSAP. This is now the only route for medical device manufacturers and medical technology companies to show compliance with the Canadian requirements for quality management systems. In order to receive a licence from Health Canada to place their products on the market, medical device manufacturers and medical technology companies must have successfully completed the MDSAP program.
MDSAP is based on the principle of “all or nothing”. If upon entry into the program a product is to be marketed in all MDSAP participating countries, the audit needs to cover all the jurisdictions. It is not possible to select only certain countries.
On the other hand, if you are not selling in all the MDSAP participating countries, it is possible to start with one jurisdiction and then extend the scope of the requirements as you move along.
On 1 January 2019, Health Canada, the Canadian Medical Devices Conformity Assessment System (CMDCAS), was replaced by MDSAP. This is now the only route for medical device manufacturers and medical technology companies to show compliance with the Canadian requirements for quality management systems. In order to receive a licence from Health Canada to place their products on the market, medical device manufacturers and medical technology companies must have successfully completed the MDSAP program.
MDSAP is based on the principle of “all or nothing”. If upon entry into the program a product is to be marketed in all MDSAP participating countries, the audit needs to cover all the jurisdictions. It is not possible to select only certain countries.
On the other hand, if you are not selling in all the MDSAP participating countries, it is possible to start with one jurisdiction and then extend the scope of the requirements as you move along.
Those working in legal and international trade departments in the medical device sector and also in quality management, including internal and external QM auditors, have to follow the MDSAP Audit Approach and should have excellent knowledge of the requirements of the program.
All the audit requirements of MDSAP are documented in the MDSAP Audit Approach. This document is available on the FDA website.
The different tasks are described in the 7 process chapters of the MDSAP:
Process Chapter 1: Management
Process Chapter 2: Device Marketing Authorization and Facility Registration
Process Chapter 3: Measurement Analysis and Improvement
Process Chapter 4: Medical Device Events and Advisory Notices Reporting
Process Chapter 5: Process: Design and Development
Process Chapter 6: Production Service and Controls
Process Chapter 7: Purchasing
The MDSAP Audit Approach describes the individual requirements of the program and it also contains explanations, links between the individual tasks and applicable regulatory requirements. This gives a good overview of the expectations which apply during the MDSAP audit.
An MDSAP audit can be combined with an (existing) CE certification or certification to ISO 13485.
History of the IMDRF
The International Medical Device Regulators Forum (IMDRF) was founded in February 2011 to discuss the future direction of regulatory harmonization in the field of medical technology. The forum is made up of voluntary groups of medical regulators from around the world and aims to accelerate the harmonization and convergence of regulations in the international medical industry.