Reprocessing of medical device including “critical C”
Ensure process compliance in your reprocessing unit for medical devices in order to fulfil standard-based and regulatory requirements.
A compliant quality management system is an essential part of quality assurance within a reprocessing unit for medical devices. It also gives the opportunity to recognise potential improvements through the use of transparent processes and to implement these in a way that adds value for your organization.
Appropriate and technically adequate reprocessing of medical devices is inseparably linked to clinic management as a whole and has a decisive impact on the quality of treatment provided for your patients. Medical devices whose reprocessing is beyond reproach from the regulatory point of view mean that liability risks are excluded and interfaces in the treatment process can interact efficiently with one another.
This is why the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM) recommend certification to DIN EN ISO 13485.
We certify your quality management system to DIN EN ISO 13485 in accordance with KRINKO/BfArM recommendations up to “critical C”. (*)




Fulfil KRINKO recommendations and gain competitive edge - the benefits of certification
A TÜV NORD CERT certificate demonstrates to your customers, stakeholders and patients, and also to your supervisory authority and the general public, that you maintain impeccable and compliant process quality for the reprocessing of medical devices based on one of the strictest quality standards in the sector.
- Certification demonstrates that your reprocessing fulfils the most stringent safety and hygiene requirements.
- You fulfil your obligations as operator as stipulated by law, based on the KRINKO/BfArM recommendations as well as the German Medical Device Operators Ordinance (Medizinprodukte – Betreiberverordnung – MPBetreibV).
- You reduce liability risks.
- Certification provides you with a competitive advantage.
- You demonstrate to your supervisory authority that you maintain impeccable and legally compliant process quality.
- Certification demonstrates your commitment to technically appropriate patient treatment and also your interest in fulfilling your duty of care towards your patients.
(* TÜV NORD CERT GmbH is accredited by the DAkkS for the certification of the reprocessing of medical devices up to and including "critical B". TÜV NORD CERT GmbH issues in-house certificates for the reprocessing of medical devices "critical C" according to the manufacturer's specifications).
Certification procedure of DIN EN ISO 13485 certification (KRINKO/BfArM)

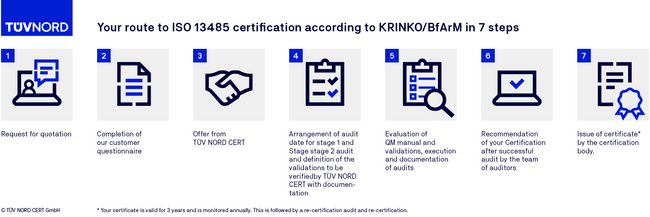
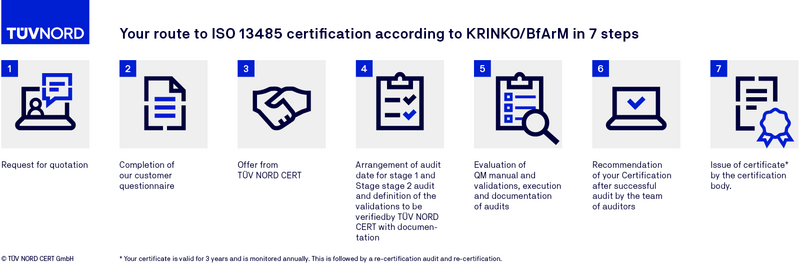
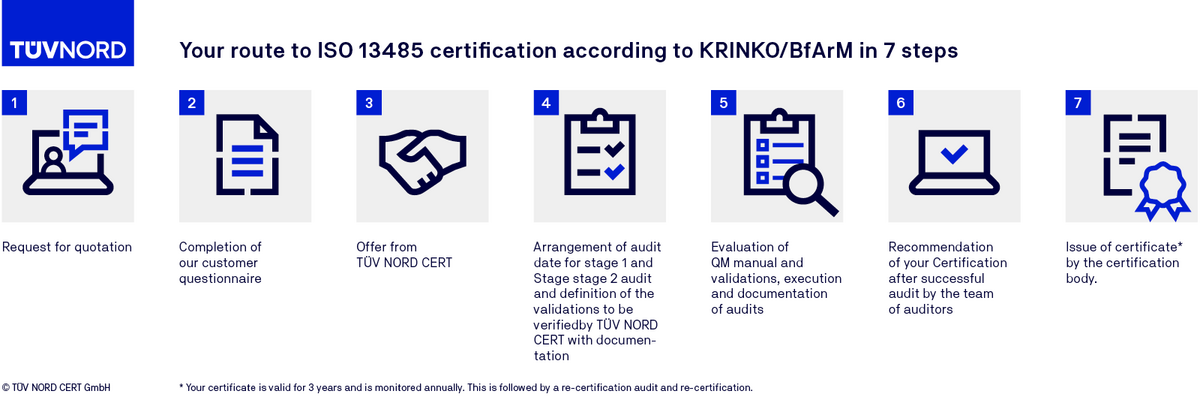
FAQs on ISO 13485 (KRINKO/BfArM)
The Robert Koch Institute regularly publishes updated guidelines which are developed by the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI). These form the basis for measures to prevent nosocomial infections (hospital infections) and combat antibiotic resistance.
The KRINKO recommendations are based on the German Protection against Infection Act (IfSG) Section 23.1 and are therefore regulatory in nature. This is particularly true of the KRINKO recommendation regarding hygiene requirements when reprocessing medical devices (Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten) as published in the Federal Health Bulletin (Bundesgesundheitsbl 2012 - 55:1244-1310 / DOI 10.1007/500103-012-1548-6) © Springer-Verlag 2012), as these were taken up into Section 8 of the German Medical Device Operators Ordinance (Medizinprodukte – Betreiberverordnung – MPBetreibV). This means that the KRINKO recommendations directly apply to the operation and use of medical devices, including necessary reprocessing.
Certification of quality management for reprocessing of medical devices is above all suitable for institutions which operate a reprocessing unit for medical devices.
The risk classification of medical devices provides for three (3) categories which are based on the risk of infection transmission via a device.
Devices are categorised as uncritical, semi-critical or critical. Critical medical devices place the most stringent demands on reprocessing, as they are intended for use in invasive procedures and therefore come into direct contact with body tissue or with bodily fluids such as blood. Instruments in the critical C category present the highest risk.